Cancer. Just the word itself sends shivers down many spines. Thankfully, science is constantly battling this disease, and new treatments are emerging all the time. One such treatment generating a lot of buzz is actinium-225 (Ac-225). But before we delve into this exciting therapy, let’s address the burning question: is Actinium-225 FDA approved?
Why Choose Actinium-225?
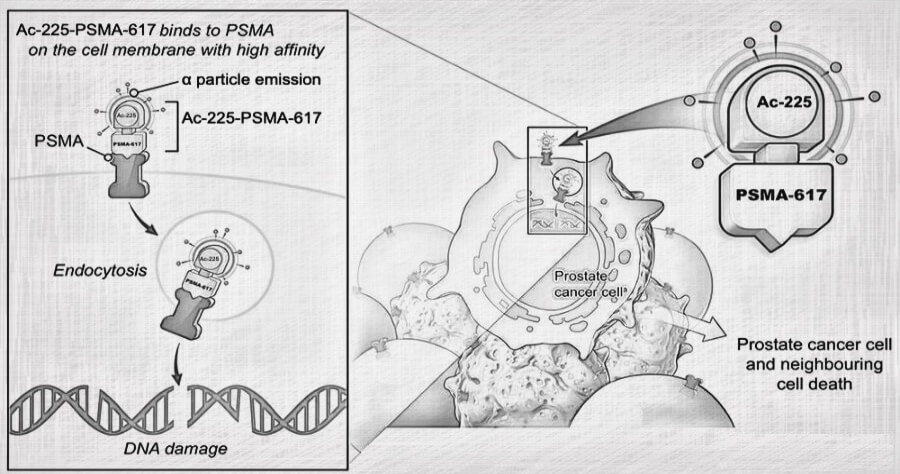
Traditional cancer treatments like chemotherapy and radiation can be effective, but they often come with harsh side effects. Actinium-225 offers a potentially more targeted approach. It’s a type of radioactive material called an alpha emitter. These emitters pack a powerful punch directly to cancer cells, leaving surrounding healthy tissue relatively unharmed.
Types of Actinium-225 Treatments
There are two main ways Actinium-225 is being explored for cancer treatment:
Targeted Alpha Therapy (TAT): Here, Actinium-225 is attached to a molecule that seeks out specific cancer cells. Once attached, the Actinium-225 delivers its radioactive payload directly to the tumor.
Actinium-225/Bismuth-213 Generators: These generators produce a continuous supply of Actinium-225. This is important because Actinium-225 has a short half-life (the time it takes for half the material to decay). Generators ensure a readily available source for treatment.
The Potential Benefits of Actinium-225
The potential benefits of Actinium-225 are quite exciting:
More Targeted: As mentioned earlier, Actinium-225 might offer a more targeted approach compared to traditional treatments, potentially reducing side effects.
Effective Against Resistant Cancers: Some cancers develop resistance to existing therapies. Actinium-225 shows promise in targeting these resistant cancers.
Treating a Variety of Cancers: Researchers are exploring Actinium-225 for treating various cancers, including prostate, brain, and leukemias.
The Road to FDA Approval
Here’s the current status of Actinium-225 and FDA approval:
While Actinium-225 itself isn’t FDA approved yet, it’s definitely on the right track. The FDA has accepted Investigational New Drug (IND) applications for certain Actinium-225-based drugs like 225Ac-FPI-2068 (April 2023). This is a crucial step because it allows for clinical trials, which are essential for gathering data on safety and efficacy.
So, the answer is: Actinium-225 isn’t directly FDA approved yet, but specific drugs incorporating it are undergoing clinical trials, paving the way for potential future approval.
FAQs
When will Actinium-225 be available for treatment?
This depends on the progress of clinical trials. It could still be a few years before Actinium-225 becomes a widely available treatment option.
Are there any side effects associated with Actinium-225?
As with any treatment, there could be side effects. Clinical trials will help determine the specific side effects and their severity.
What cancers could be treated with Actinium-225?
Researchers are exploring its use for various cancers, but more research is needed to determine its effectiveness for specific types.
Conclusion
Actinium-225 holds immense promise as a new weapon in the fight against cancer. While it’s not yet FDA approved, the ongoing clinical trials are a positive step towards making it a viable treatment option. With continued research and development, Actinium-225 could soon become a beacon of hope for many cancer patients.